Abstract
Background: MCL is a rare and incurable disease representing 5-10% of all non-Hodgkin lymphoma cases. Although intensive chemotherapy induction and up-front ASCT can induce durable remissions in fit patients, toxicity can be significant. R-DHAOx chemoimmunotherapy provides adequate outcomes and a favourable toxicity profile compared to some other induction regimens (Le Gouill S Blood 2017). The highly selective Bruton tyrosine kinase inhibitor (BTKi) acalabrutinib has minimal off-target activity and proven efficacy in relapsed MCL (Wang M Lancet 2018), however, direct combination of BTKi & chemoimmunotherapy is toxic. Thus, it is postulated that up-front use with an acalabrutinib 'window' before chemoimmunotherapy, followed by maintenance after intensive chemotherapy will prolong time to next treatment and reduce overall treatment toxicity (Kuruvilla J Hematol Oncol 2017).
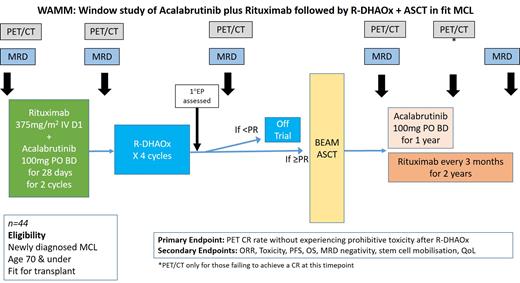
Methods: The WAMM study is an ALLG-sponsored investigator-initiated multicentre single-arm phase 2 trial (ACTRN1269000990123). Eligible patients are aged 18-70 years with previously-untreated histologically-proven CD20-positive stage II-IV MCL (all subtypes), ECOG 0-1, and no comorbidities precluding ASCT. Patient with major contraindications to BTKi use are excluded. Treatment is delivered in a unique and innovative acalabrutinib 'sandwich' design to avoid excess toxicity of combination acalabrutinib-chemoimmunotherapy (see figure). All patients receive 2 cycles of induction rituximab (R) 375 mg/m 2 IV (day 1) & acalabrutinib (A) 100mg BD PO daily (continuous) 4-weekly, followed by 4 conventional R-DHAOx chemoimmunotherapy cycles. Patients then undergo positron emission tomography (PET) assessment. Those with partial or complete response will proceed to carmustine, etoposide, cytarabine and melphalan (BEAM) autograft which is followed by A+R maintenance (A; 100 mg BD continuous for 1 year & R; 375 mg/m 2 IV, 3-monthly x 8 cycles). Patients with stable or progressive disease will be taken off study. A minimum of 3 years of post-treatment follow-up is planned.
Regular clinical, laboratory, PET, and molecular minimal residual disease (MRD) assessments will be performed. The composite primary endpoint includes safety (defined by lack of prohibitive toxicity) and complete metabolic response rate of AR induction followed by R-DHAOx chemoimmunotherapy. Prohibitive toxicity is defined as > grade 3 toxicity causing treatment cessation or major delay. Secondary endpoints include response rates, overall toxicity, overall and progression free survival, MRD negativity rates and standardised quality of life scores. All PET centres are Australasian Radiopharmaceutical Trials network (ARTnet) accredited. PET & MRD analyses are centralized. An extensive exploratory biomarker substudy is planned. The sample size is 44 according to a Simon's 2-stage design. If 3 or more positive responses without prohibitive toxicity are seen in the first 14 patients, 30 further patients will be recruited. The trial has currently enrolled 20 patients from 11 Australian sites.
Acknowledgements: Acalabrutinib and a research grant has been supplied by Astra Zeneca. Maintenance Rituximab has been supplied by Sandoz. Olivia Newton-John Cancer Research Institute Haematology biobank.
Hawkes: Merck KgA: Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees; Specialised Therapeutics: Consultancy; Bristol Myers Squib/Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Other: Travel and accommodation expenses, Research Funding, Speakers Bureau; Janssen: Speakers Bureau; Regeneron: Speakers Bureau; Antigene: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Merck Sharpe Dohme: Membership on an entity's Board of Directors or advisory committees. Wight: Jannsen: Honoraria, Other: Travel subsidies; Abbvie: Honoraria, Other: Travel subsidies. Cheah: Novartis: Consultancy, Honoraria; Beigene: Consultancy, Honoraria; TG therapeutics: Consultancy, Honoraria; Lilly: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria; Ascentage Pharma: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; MSD: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Abbvie: Research Funding; BMS: Consultancy, Research Funding; Roche: Consultancy, Honoraria, Other: travel, Research Funding. Ku: Roche: Consultancy; Genor Biopharma: Consultancy; Antegene: Consultancy. Opat: Merck: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astra Zeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BeiGene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Research Funding; Sandoz: Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; CSL Behring: Honoraria, Membership on an entity's Board of Directors or advisory committees; Monash Health: Current Employment. Devitt: Oncosec: Consultancy; Gilead Sciences: Consultancy; Merck Sharp and Dohme: Honoraria; Eisai: Honoraria; Amgen: Honoraria. Fong: Amgen, BMS: Speakers Bureau; Amgen: Research Funding; AbbVie, Amgen, Novartis, Pfizer, Astellas: Honoraria. Barraclough: Roche: Honoraria, Other: Conference sponsorship.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal